Novel insight into protein phosphatase substrate recognition published by the Nilsson group in Molecular Cell
Protein phosphatases play important roles in cellular signaling as they remove phosphorylations from proteins hereby counterbalancing kinase activity. Aberrant cellular signaling is the cause of most human diseases and understanding the basic principles of signal regulation is of utmost importance. Understanding the role of protein phosphatases has been challenging, as the mechanism of substrate recognition is unknown. Now research in the lab of Associate Professor Jakob Nilsson have uncovered how one of the major phosphatases, PP2A-B56, achieves substrate specificity hereby providing insight into regulation of numerous signaling pathways in cells.
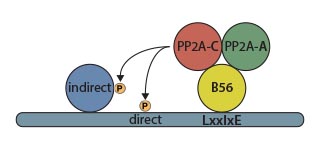
Figure: Illustration showing how the identified LxxIxE motif targets the PP2A-B56 phosphatase to substrates and regulators
PhD student Emil Hertz and Postdoc Thomas Kruse investigated how PP2A-B56 bound to different substrates that they had identified through mass spectrometry in collaboration with Professor Jesper V. Olsens group also from CPR. They realized that a common short consensus motif was present in all of the substrates and that this was responsible for binding to the B56 regulatory subunit of the complex. “This was quite exiting as our work identified the first general principles for how PP2A, the major protein phosphatase, recognizes its substrates” says Emil Hertz. In collaboration with Professor Guillermo Montoyas group the structural and biophysical aspects of B56 binding to the consensus motif was investigated revealing a fully conserved binding pocket on B56. “Our work shows that the consensus motif has been conserved through more than 1 billion years of evolution revealing an ancient mechanism of substrate recognition by PP2A-B56” explains Thomas Kruse. An interesting aspect of the study is that small changes in the amino acid composition of the consensus motif profoundly influence the strength of the binding to B56 and thus substrate phosphorylation status. “We found that the motif strength in substrates is carefully adjusted to balance the corresponding kinase activity in a given pathway and this is important for proper cellular signaling” says Jakob Nilsson.
Future studies are aimed at understanding how PP2A-B56 regulates different signaling pathways through binding the consensus motif and understanding how this might be deregulated in different human diseases. The hope is that this can help pinpoint diseases where inhibition of PP2A-B56 can be beneficial.
Read the paper in Molecular Cell: A Conserved Motif Provides Binding Specificity to the PP2A-B56 Phosphatase
Authors
Emil Peter Thrane Hertz (CPR)
Thomas Kruse (CPR)
Norman E. Davey
Blanca López-Méndez (CPR)
Jón Otti Sigurðsson (CPR)
Guillermo Montoya (CPR)
Jesper V. Olsen (CPR)
Jakob Nilsson (CPR)
