Nielsen group details the consequences of biotin deprivation in metabolic disorders using high-res mass spectrometry
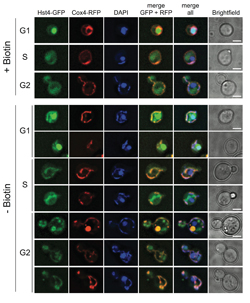
Hst4p translocates to mitochondria upon biotin deprivation
The essential vitamin biotin is a covalent and tenaciously attached prosthetic group in several carboxylases that play important roles for cellular growth and regulation of energy metabolism.
Cellular biotin starvation is known to cause inefficient energy expenditure, ultimately leading to a state of metabolic disorder. However, the molecular details surrounded the cellular role of biotin are not fully understood.
In this research article, the Nielsen group in collaboration with researchers from Novo Nordisk and the Novo Nordisk Foundation Center for Metabolic Research finds that biotin starvation causes a major energy switch by shuttling acetyl-CoA into the mitochondria, which results in mitochondrial protein hyperacetylation reminiscent of SIRT3 inhibition in human cells.
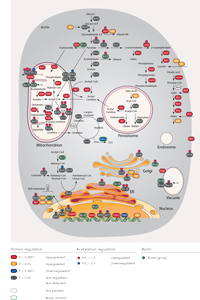
The cellular consequences during biotin starvation
Using high-resolution mass spectrometry the presented study demonstrate that reorientation of the nucleocytosolic acetyl-CoA pool during biotin scarcity manifests itself into mitochondrial hyperacetylation, and causes wide-spread downstream effects upon cellular respiration and redox balance. The observed responses are reminiscent of mammalian cells lacking the mitochondrial protein SIRT3, consistent with previous studies demonstrating that biotin deprivation is inversely related to cellular energy status, resulting in increased insulin sensitivity. Moreover, oxidative stress contributes to the progression of various human diseases such as type 2 diabetes, where an increased supply of energy substrates results in excessive mitochondrial ROS. As a result, the data presented by the Nielsen group entails a valuable resource that details the link between biotin starvation and ROS production in eukaryotic cells, and delineates the effects of biotin availability upon insulin sensitivity through acetyl-CoA flux and mitochondrial hyperacetylation.
With biotin deficiency being involved in various metabolic disorders, this study provides valuable insight into the metabolic effects biotin exerts on eukaryotic cells.
Read the full article in Nature Communications
 Authors: Christian T. Madsen, Kathrine B. Sylvestersen, Clifford Young, Sara C. Larsen, Jon W. Poulsen, Marianne A. Andersen, Eva A. Palmqvist, Martin Hey-Mogensen, Per B. Jensen, Jonas T. Treebak, Michael Lisby, and Michael L. Nielsen (Authors from Novo Nordisk Foundation Center for Protein Research in bold)
Authors: Christian T. Madsen, Kathrine B. Sylvestersen, Clifford Young, Sara C. Larsen, Jon W. Poulsen, Marianne A. Andersen, Eva A. Palmqvist, Martin Hey-Mogensen, Per B. Jensen, Jonas T. Treebak, Michael Lisby, and Michael L. Nielsen (Authors from Novo Nordisk Foundation Center for Protein Research in bold)
Novo Nordisk Foundation Center for Protein Research, University of Copenhagen. The center is supported financially by the Novo Nordisk Foundation (Grant agreement NNF14CC0001).

