The Mailand group has discovered a new human protein, DVC1, crucial for protection of genome stability:
All cells suffer numerous daily DNA damaging insults, which may alter the content and organization of the genome, jeopardizing cell and organismal survival and fitness. The genome is particularly vulnerable to DNA damage during DNA replication, where DNA is precisely duplicated as part of the cell division process. The encounter with damaged DNA blocks ongoing replication, invoking a serious threat to genome stability as the stalled replication machinery may collapse and give rise to highly cytotoxic DNA double-strand breaks. To mitigate such danger, cells have evolved damage avoidance strategies to enable bypass of replication-blocking lesions. The predominant damage bypass mechanism in mammalian cells involves specialized low-fidelity translesion synthesis (TLS) DNA polymerases, which are able to replicate damaged DNA and thus allow the replication machinery to bypass the lesion. The TLS process tends to be error-prone, however, and accordingly it must be kept tightly in check to prevent excessive mutagenesis of the genome. The small modifier protein ubiquitin is known to play a central role in coordinating the TLS process with normal DNA replication, but our understanding of this involvement is still limited. 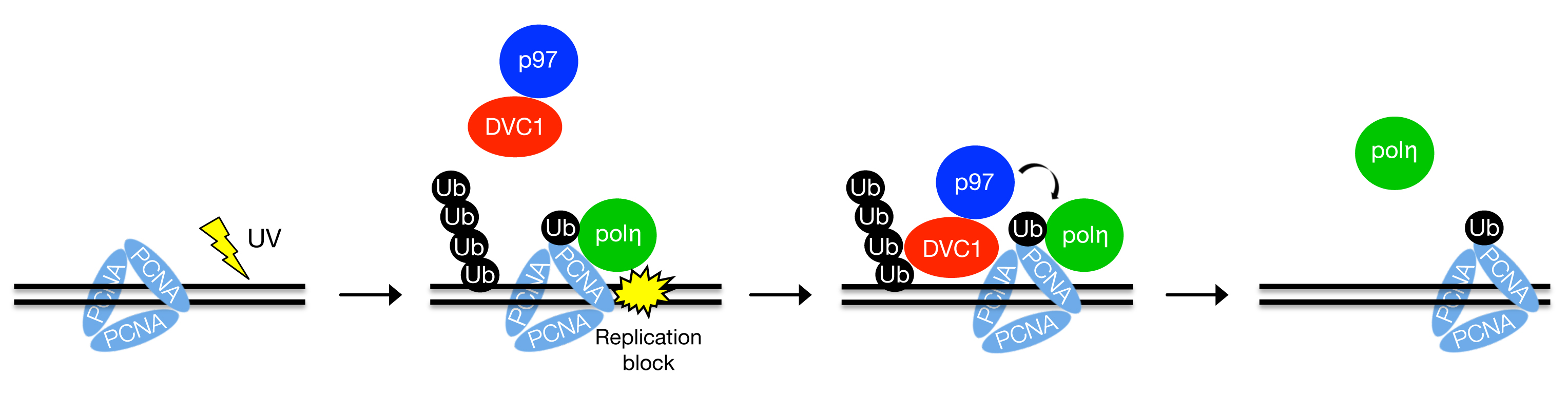
In a new study published in Nature Structural and Molecular Biology, the Mailand group at CPR identified a new human protein, DVC1, which plays an important role in the ubiquitin-dependent signaling responses that protect genome stability following replication problems. Mechanistically, DVC1 functions as an adaptor for the ubiquitin-selective chaperone protein p97, promoting its accumulation at DNA damage-stalled replication forks. Following its DVC1-dependent recruitment to the blocked replisome, p97 then promotes the displacement of TLS polymerases from this structure, providing a means for cells to restrain the association of TLS polymerases with the replication machinery. Loss of DVC1 function selectively sensitizes cells to DNA damaging agents that elicit replication blocks and enhance the mutation rate induced by such agents. The authors also demonstrate that the key role of DVC1 in protecting genome integrity following replication problems is evolutionarily conserved between humans and lower eukaryotes such as the worm C. elegans. These findings establish DVC1 as a DNA damage-targeting p97 adaptor that protects cells from the deleterious consequences of replication blocks, and provide important new mechanistic insight into how ubiquitin-mediated processes regulate cellular responses to DNA damage. The study is published in Nature Structural & Molecular Biology.
