DNA Repair's Hidden Cost: Why 'Fixed' Cells Never Fully Recover
Our DNA breaks thousands of times daily—even a simple walk in sunlight causes extensive damage. While evolution has equipped cells with powerful repair mechanisms to fix these breaks, new research reveals that successful DNA repair comes with an unexpected price: lasting changes to the DNA’s three-dimensional structure and function.
The price cells pay for successful DNA repair
DNA doesn't exist as a simple linear molecule. Instead, it lives in synergy with thousands of proteins and RNA species to form a complex three-dimensional (3-D) structure called chromatin—allowing two meters of DNA to strategically fold into a nucleus just 8 micrometers wide. It was a tricky task for evolution to make this happen, but nature is smart and took advantage of its own success. The intricate 3-D chromatin folding is used to bring important functional regulators and their targets to close proximity and thus enable proper gene regulation and cellular function.
However, our genetic information is constantly challenged by assaults from environment and our own metabolism that can damage – in extreme cases even break – the DNA molecule. The repair of DNA as such has been long studied and is now incredibly well understood and appreciated (including the Nobel Prize in Chemistry in 2015 for DNA repair founders). However, an important question is left unanswered: What happens to the intricate 3-D structure of the chromatin surrounding the broken DNA? And even more importantly, how this affects the function of neighboring genes not only when DNA breaks, but also after it is repaired? In other words, we do not know whether nature also possess an analogous mechanism to restore its 3-D chromatin folding and thus restore full functionality.
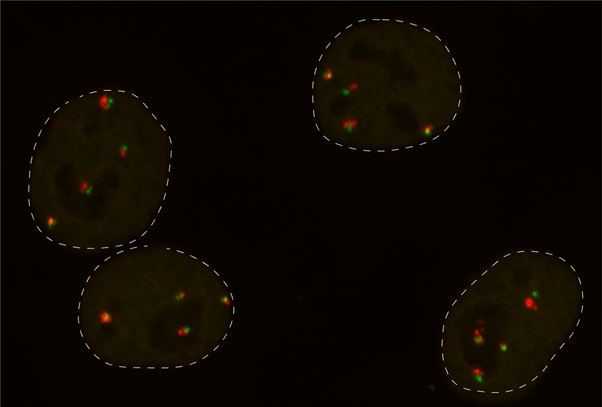
To address these questions, the researchers at the Novo Nordisk Foundation Center for Protein Research at the University of Copenhagen (CPR) and The Danish Cancer Institute (DCI) set out to track cells with defined DNA breaks generated by the CRISPR/Cas9 technology used in experimental as well as therapeutical genome editing. They managed to do so thanks to combining an unusually broad spectrum of state-of-the-art technologies including high content microscopy, transcriptome analysis, 3-D techniques like DNA FISH and Micro-C, RNA FISH, genomics and high content live cell imaging.
The outcome of their research was indeed rewarding but at the same time highly unexpected. They found that even after successful DNA repair has been accomplished, the surrounding chromatin did not re-fold exactly to the same 3-D shape as it was before the DNA breakage occurred. These structural changes were accompanied by changes in gene expression and weakened the ability of the affected cells to efficiently react to external stimuli. Most surprisingly, these changes persisted on the post-repair chromatin and were even passed to daughter cells, creating lasting consequences to cell physiology. So, against all expectations, evolution did not seem to coordinate repair of DNA breaks (which in most cases is timely and efficient) with similarly efficient mechanisms that would faithfully restore the 3-D chromatin folding in rather large segments surrounding repaired DNA lesions.
Susanne Bantele, the principal investigator compares the discovery to many hours sailing on the sea, reliant only on her own ability to navigate safely back to the harbor:
“Consider a sailboat. Its ability to stay on course depends on the precise shape and tension of its sails. If ropes become tangled, the sails shift, and the boat drifts off track. Similarly, when chromatin folding is disturbed—even slightly—the cell’s transcriptional “course” changes, steering it toward altered physiology. Over time, like a boat weathered by countless storms, cells accumulate these structural changes through repeated DNA damage and repair cycles. This leads to decreased cellular fitness and responsiveness to occasional but inevitable ‘storms’ from environment as well as those generated by cells’ own metabolic activities, leaving behind a phenomenon we call "chromatin fatigue".
Jiri Lukas, the head of the Chromosome Stability and Dynamics Group adds:
“One of the biggest surprises in the whole project was when we noticed that chromatin fatigue is passed from one cell generation to the other. Given the trillions of cell divisions that occur during the life span in a single human body, it is conceivable that even slow but steady cumulation of chromatin fatigue can at some point become the “last drop in a full glass” to spill over and thus derail the course of previously normal cells towards uncontrolled proliferation, a hallmark of devastating diseases including cancer.”
Rethinking DNA Repair in the Age of Gene Editing
The discovery of chromatin fatigue challenges the long-held dogma that DNA sequence mutations are the sole consequence of DNA breaks that can reprogram the cells and thus cause disease. But the implications of this work go ever further as Susanne Bantele explains:
“Our discovery of chromatin fatigue raises important questions about gene editing technologies and therapies that intentionally break DNA, suggesting we need to consider not only whether we successfully introduced the desired modification to the targeted DNA, but also how the accompanying repair potentially influenced the function of other gens within the neighboring chromatin landscape.”
In summary, the finding that DNA repair leaves lasting impairments to chromatin structure and function represents a paradigm shift in our understanding of cellular aging, cancer development, and the true cost of maintaining genomic integrity. It also opens avenues for future research by posing important and previously concealed questions such as: Which classes of genes are specifically vulnerable for chromatin fatigue? Which mechanisms drive refolding of the genomic DNA after DNA repair and why they fail to work with 100% fidelity? How can we best use the CRISPR/Cas9 technology in experimental and medical gene editing without having to cope with the side-effects of chromatin fatigue?
This research was conducted by Susanne Bantele and Jiri Lukas (Novo Nordisk Center for Protein Research, Department of Cellular and Molecular Medicine, University of Copenhagen) in collaboration with Anja Groth and Nils Krietenstein (Danish Cancer Institute, Department of Cellular and Molecular Medicine, University of Copenhagen).
Read the full story in Science here https://tinyurl.com/5n6zw3ye and check our Bluesky Tweetorial https://bsky.app/profile/sbantele.bsky.social/post/3m4yogrvmqs2a.
