Rationale for combination therapy in acute myeloid leukemia
Acute myeloid leukemia (AML) is a highly heterogeneous disease for which there is an unmet need for improved targeted therapies. Selinexor has shown promise for treatment of AML but selection of patient responders is challenging and resistance to selinexor is not well understood. In a new study, the Olsen Group and collaborators use subcellular phosphoproteomics to devise rational combination therapies to enhance selinexor treatment efficacy in responders and overcome resistance in non-responders.
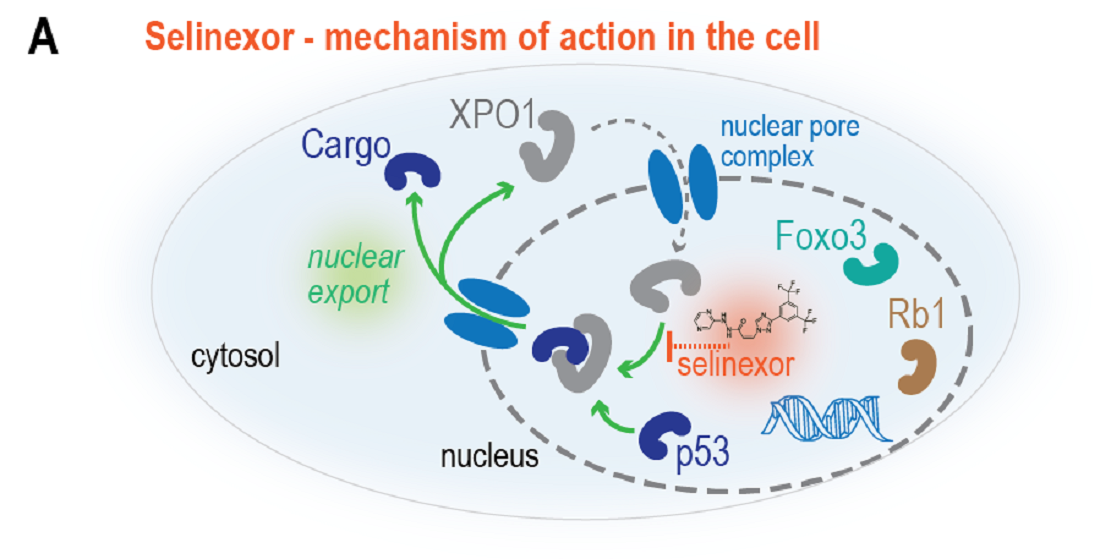
In human cells, selinexor targets the exportin-1 (XPO1) protein, which controls the transport of tumor suppressor proteins such as p53 and Rb1 in and out of the cell nucleus. Inhibition by selinexor traps these proteins in the nucleus and thereby enhances their tumor suppressor activity (illustration A). Consequently, selinexor is emerging as a promising drug for cancer therapy, especially in leukemia, and has been tested in several clinical trials. As single agent activity of selinexor has proven limited, there is an unexplored potential for combination therapy with other targeted treatments.
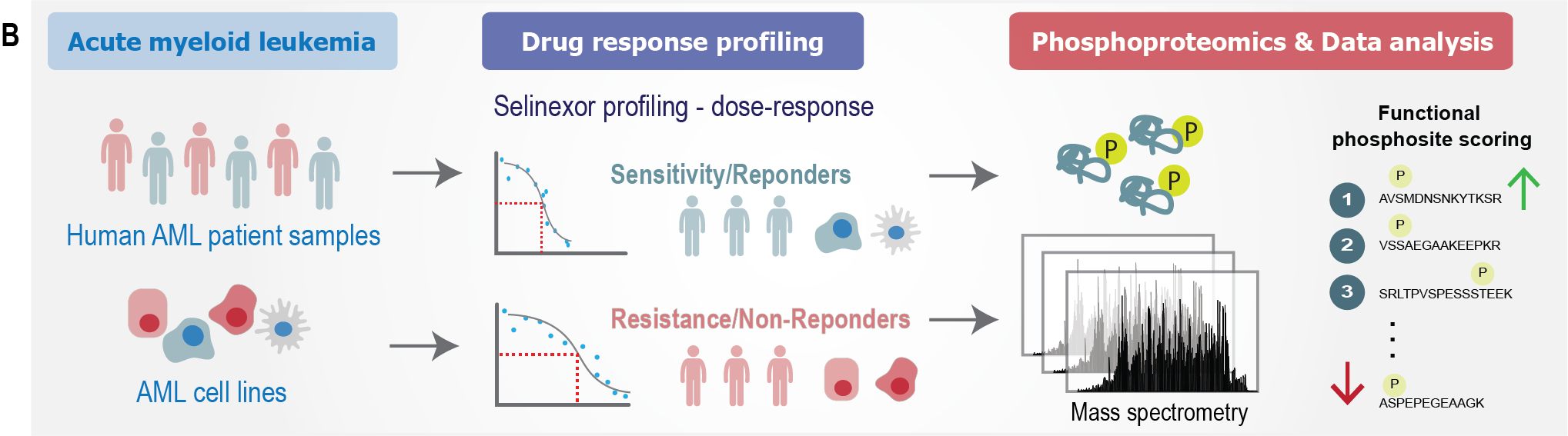
In a new study, the Olsen group at Novo Nordisk Foundation Center for Protein Research (CPR), University of Copenhagen, used their world-leading expertise in mass spectrometry-based proteomics to understand the molecular reasons why some patients respond to treatment with selinexor, while others do not. In collaboration with Saez-Rodriguez lab in Heidelberg, Germany, and Acrivon Therapeutics in MA, USA, they profiled samples from patients with AML in order to identify rational drug combinations that could potentially increase patient sensitivity to selinexor and overcome resistance (illustration B).
In selinexor-sensitive AML cells, the research team found that increased levels of wildtype p53 enhances selinexor efficacy. They successfully demonstrated this by co-treatment of cells with the drug nutlin-3a, which prevents p53 degradation. This finding suggests that combination with nutlin-3a may increase the effect of selinexor treatment in AML patients with the relevant molecular responder profile (illustration C).
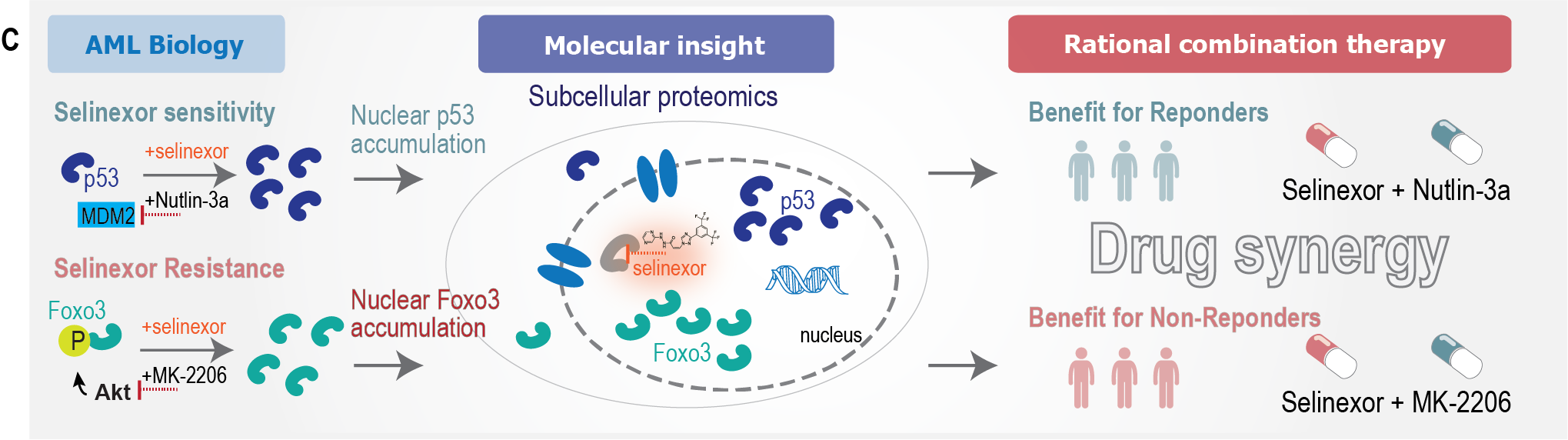
Importantly, the research team also analysed selinexor-resistant AML cells to find molecular pathways causing the lack of selinexor effect. Here, they discovered dysregulation of the AKT-Foxo3 signaling axis whereby phosphorylated Foxo3, an important tumor suppressor, was retained in the cytoplasm and therefore could not exert its function as transcription factor in the nucleus. The researchers showed that treatment with the drug MK-2206 in combination with selinexor could overcome resistance. The synergistic drug combination of selinexor and MK-2206 resulted in nuclear accumulation of Foxo3 and enhanced cell killing (illustration C).
With this study, two new combinations of drugs are proposed; one for selinexor responders and one for selinexor non-responders. ’Our study provides novel rational drug combinations to enhance sensitivity of selinexor and overcome drug resistance, both of which are not possible to predict from genetic analysis of patients. This really highlights the importance of our proteomic analyses. We hope that our findings can be translated in the clinic to benefit AML patients in the future’ says Professor Jesper Olsen from CPR.
The study Phosphoproteomics of primary AML patient samples reveals rationale for AKT combination therapy and p53 context to overcome selinexor resistance was published in Cell Reports.
Contact
Professor Jesper Velgaard Olsen
+45 35 32 50 22
jesper.olsen@cpr.ku.dk
