‘DNA replication in a tube’ provides new insights into chromatin assembly
The cells in our body regularly divide to produce new cells. Before a cell divides, it needs to duplicate its DNA to ensure that the new cells will contain the same genetic information. This occurs during the process of DNA replication. In addition, the cell needs to replicate the packaging system of DNA, called chromatin. The chromatin packaging system enables two meters of DNA to fit into the cell nucleus and consists of DNA wrapped around proteins named histones. Apart from its role in tightly packing the DNA, chromatin regulates which parts of the DNA are active in the cell, thereby determining how the cell behaves. As the cell divides, it is therefore essential that not only the DNA code is faithfully replicated, but the chromatin information as well. Otherwise, the newly formed cells will start to malfunction, which can lead to diseases such as cancer.
Taking chromatin replication out of the cell
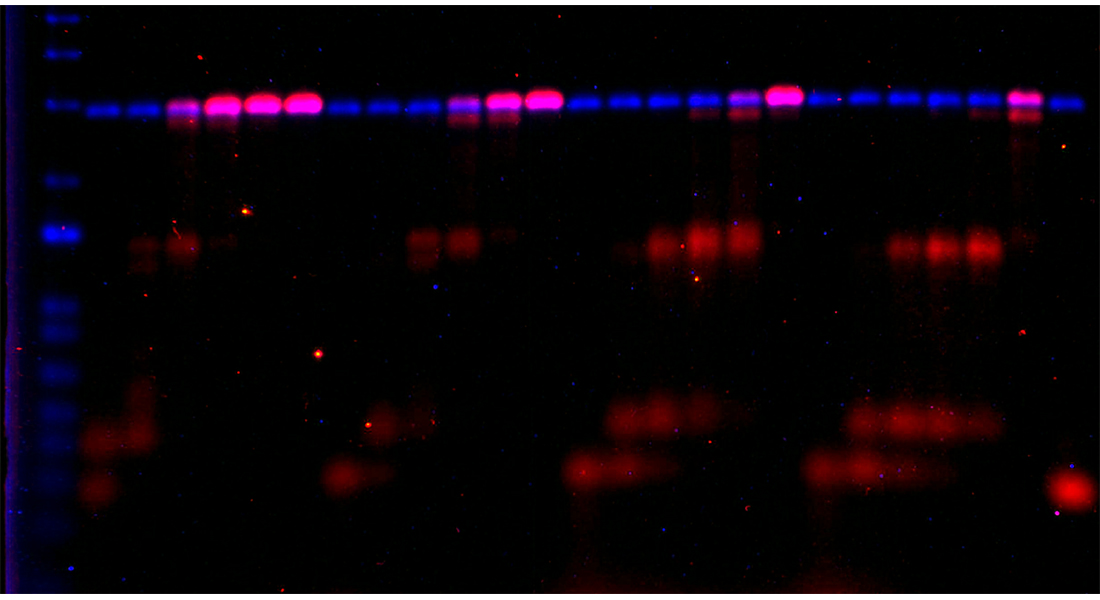
While DNA replication has been studied extensively, chromatin replication is much more challenging to investigate. This is due to the complexity of chromatin structure and its many interconnected functions in cells. As a consequence, the mechanisms behind this process remain largely unknown. In order to change this, the researchers used a variety of biochemical systems to re-create the DNA replication and chromatin assembly of yeast in a test tube. Co-first author Clément Rouillon (Hubrecht Institute) explains: “Within cells, there are many back-up mechanisms and checkpoints to ensure that essential processes like chromatin replication are carried out correctly before the cell divides. This allows the cell to correct errors in these processes, but it can mask the function of individual molecules. Therefore, we took the process of chromatin assembly outside the complex cellular environment. For this we combined new in vitro chromatin assembly assays with powerful DNA replication approaches previously developed by the Diffley and Yeeles laboratories in the UK. This provides a highly controlled system which allows us to carefully study the process of chromatin assembly, and its link to the large DNA replication machinery which includes over 50 different proteins.”
Asymmetrical crosstalk
By using these systems, the researchers discovered that chromatin assembly occurs in a different manner on each of the two replicated DNA strands. Co-first author Bruna Eckhardt (Hubrecht Institute) explains: “During DNA replication, the two strands of DNA can be distinguished as the leading and lagging strand. The leading strand is made continuously, meaning that the DNA is replicated in one piece. The lagging strand is produced in small pieces that are connected afterwards. We already knew that the leading and lagging strand contain different proteins. What we discovered now is that this leads to differences in the way the chromatin machinery affects DNA replication.” The team specifically focused on the protein CAF-1. This so-called ‘histone chaperone’ binds histones to deliver them to newly formed DNA, thereby forming the chromatin packaging system. They show that CAF-1 indeed makes chromatin on both DNA strands. However, the interplay between CAF-1 and the enzymes involved in DNA replication differs between the two strands. Rouillon: “Our results suggest that DNA replication and chromatin assembly cannot occur simultaneously on the leading strand, while the two processes might be coupled on the lagging strand. This is consistent with the fact that DNA replication is in itself an asymmetrical process, with differences between the two DNA strands.
Chromatin assembly as a therapeutic target
The results of the study are surprising, because the processes of DNA replication and chromatin assembly were thought to occur independently before. “Our data highlight that there is in fact a functional interaction between the two, meaning that affecting one will influence the other,” says group leader Francesca Mattiroli (Hubrecht Institute). Not only do these results shed new light on DNA and chromatin replication, fundamental processes which we cannot live without, they also give new directions for cancer research. Mattiroli: “These processes are often deregulated in cancer cells, and we know that CAF-1 is involved in the progression of several types of cancer. Therefore, understanding the normal function of CAF-1 will bring us closer to developing new cancer treatments that target this protein.”
The study was published in Nucleic Acids Research – find the paper here.
Contact: Anja Groth (e-mail: anja.groth@cpr.ku.dk)
