Phosphoproteomics of primary patient cells reveals CDK7 kinase as a new therapeutic target in ovarian cancer
Ovarian cancer is the most lethal gynecological malignancy in the western world as it is generally diagnosed at late stages due to lack of specific symptoms, and because most patients develop resistance to chemotherapy. This is in part due to the lack of good experimental models that recapitulate the complex biology of the disease. For instance, the cellular origin of high-grade serious ovarian cancer - the most aggressive and diffuse type of ovarian cancer - is still controversial. Thus, the use of clinically relevant models has emerged as a prerequisite to gain significant insights into this devastating neoplasm.
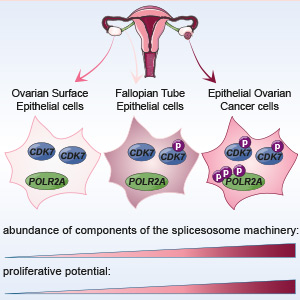
Figure 1: Using mass spectrometry-based proteomics, Francavilla et al., discovered that the kinase CDK7 and its substrate POLR2A is activated in epithelial ovarian cancer (EOC) but not healthy ovarian epithelial cells.
In this study we analyzed ex-vivo cultured primary patient cells and performed quantitative phosphoproteomics to compare the cellular phospho-signaling networks of Epithelial Ovarian Cancer (EOC) with its healthy counterparts, Ovarian Surface Epithelium (OSE) and the Fallopian Tube Epithelium (FTE), respectively.
Our approach defines the molecular landscape of EOC, paving the way for efficient therapeutic approaches for patients. We uncovered cancer-specific protein expression profiles and phosphorylation signatures and found that the kinase CDK7 phosphorylates POLR2A and drives EOC cell proliferation. Furthermore, we highlight the potential of phosphoproteomics to identify clinically relevant and druggable pathways in cancer.
New potential therapeutic target for treatment of ovarian cancer
Our study shows that the specific pharmacological inhibition of CDK7 kinase activity leads to growth arrest in different ovarian cancer cells, but not in cancer cells of different origin. CDK7 is therefore a new potential therapeutic target for the treatment of ovarian cancer.
Our journey towards our conclusions
To elucidate ovarian cancer-specific signaling networks, we made use of a unique combination of quantitative phosphoproteomics and proteomics on patient-derived primary epithelial cells using state-of-art mass spectrometry. We then validated our findings by immunohistochemistry, tissue microarrays (TMA) analysis and cell-based assays. This multi-disciplinary approach allowed us to elucidate the cellular protein signaling signatures underlying ovarian cancer cells compared to their healthy counterparts.
We hope to make a difference in the treatment of ovarian cancer
There is currently an urgent unmet need of new biomarkers and therapeutic targets in ovarian cancer. We provide both of these with our study here by using mass spectrometry-based quantitative phosphoproteomics of primary cells to identify ovarian cancer specific phosphorylation patterns that can serve as a biomarker signature and at the same time present the CDK7 kinase as new therapeutic target.
Link to article in Cell reports: ‘Phosphoproteomics of Primary Cells Reveals Druggable Kinase Signatures in Ovarian Cancer’.
List of authors from Novo Nordisk Foundation Center for Protein Research (CPR)
Chiara Francavilla
Kalliopi Tsafou
Rosa Rakownikow Jersie-Christensen
Søren Brunak
Lars J. Jensen
Jesper V. Olsen
