Novel principle for major cell signaling mechanism published by Nilsson group in Nature Cell Biology
Researchers from the Nilsson group at Novo Nordisk Foundation Center for Protein Research have revealed a novel principle for cellular signaling pathways regulated by protein phosphatases. Reversible phosphorylation of proteins on serine and threonine residues constitutes the major signaling mechanism in cells and is essential for numerous processes throughout the life cycle of a cell, including cell division. Since signaling by phosphorylation is often deregulated in human disease it is of utmost importance to uncover the core principles of its regulation in order to understand the underpinnings of disease progression.
Temporal regulation of signaling events
Until now, phosphorylated threonine and serine residues have commonly been viewed as equivalent and interchangeable post-translational modifications but work from the Nilsson lab now reveals that they act as distinct post-translational modifications. The researchers show that this is due to phosphothreonine being dephosphorylated faster than phosphoserine resulting in distinct temporal behavior of the two post-translational modifications. Importantly, the distinct kinetics in dephosphorylation is used to control the temporal order of signaling events in cells.
Cell division as a model
The researchers used cell division as a model for studying temporal regulation of phosphorylation-mediated signaling. During mitosis (division of the cell nucleus) the Cdk1-cyclin B1 kinase complex phosphosphorylates thousands of proteins on phosphothreonine (TP) and phosphoserine (SP) sites to orchestrate the segregation of chromosomes. Upon exit from mitosis these sites are dephosphorylated by the protein phosphatase 2A (PP2A) and through biochemical and cellular assays the researchers found that TP sites are rapidly dephosphorylated while SP sites are slowly dephosphorylated. This difference is used to coordinate the temporal activation of the anaphase-promoting complex (APC/C), the major regulator of mitotic exit, as well as components involve in cytokinesis. A striking example is that mutating a single threonine to serine in the protein INCENP slowed dephosphorylation and resulted in a complete failure of cytokinesis because INCENP remained bound to chromosomes.
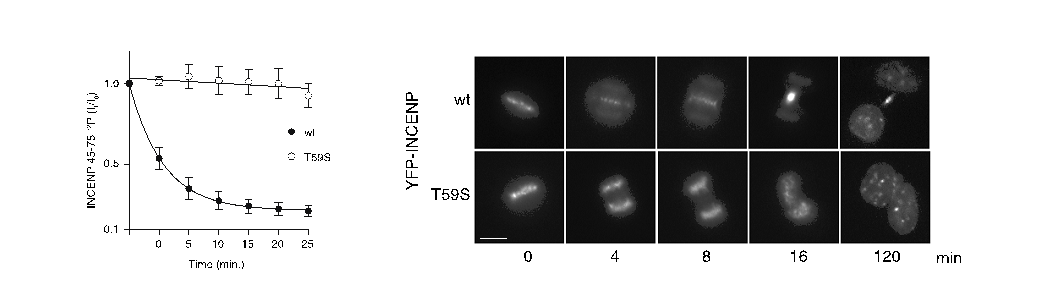
In vitro dephosphorylation of INCENP and INCENP T59S (left) reveals that the serine mutation slows dephosphorylation and in vivo this prevents INCENP translocation from chromosomes and cytokinesis (right).
General mechanism
Although the work focused on PP2A regulation of cell division, the principle uncovered is likely general and applies to all phosphorylation-mediated signaling cascades regulated by PP2A complexes and potentially other protein phosphatases. “The preference of a protein phosphatase for either phosphothreonine or phosphoserine may constitute a simple yet general mechanism for providing temporal resolution of events initiated by the same signal in many cellular signaling networks regulated by phosphorylation” says Professor Jakob Nilsson who headed the study. The Nilsson group is now investigating how the uncovered principle is used to orchestrate other major signaling pathways in the cell regulated by protein phosphatases.
Potential benefits
“The fundamental importance of protein phosphorylation in all aspects of biology has been recognized for decades but it is only recently that we start to understand the detailed principles of how dephosphorylation is regulated” says Jakob Nilsson whose group last year published how protein phosphatases recognize their substrates through a specific binding motif. “These discoveries fill in some important gaps in our knowledge. They have great potential to improve our means of disease intervention because they help us understand how specificity is obtained in this fundamental signaling pathway thus aiding the development of inhibitory drugs against specific phosphatases in order to moderate cellular processes that are deregulated in disease” adds Jakob Nilsson.
Read the full article in Nature Cell Biology: Distinct kinetics of serine and threonine dephosphorylation are essential for mitosis
Authors (CPR authors underscored)
Jamin B. Hein, Emil P. T. Hertz, Dimitriya H. Garvanska, Thomas Kruse & Jakob Nilsson
